Cone 5 reduction mug left and cone 6 oxidation mug right These are made from Plainsman M340 and have transparent glazes. By contrast reduction firing tends to create rich organic earthy colors.
Oxidation And Reduction Atmospheres Coloring Pages - If you're searching for video and picture information related to the key word you've come to pay a visit to the ideal site. Our website gives you hints for seeing the highest quality video and image content, hunt and find more enlightening video articles and graphics that fit your interests. comprises one of tens of thousands of movie collections from various sources, particularly Youtube, therefore we recommend this video for you to view. It is also possible to bring about supporting this website by sharing videos and graphics that you enjoy on this site on your social networking accounts such as Facebook and Instagram or educate your closest friends share your experiences concerning the ease of access to downloads and the information you get on this site. This site is for them to visit this website.
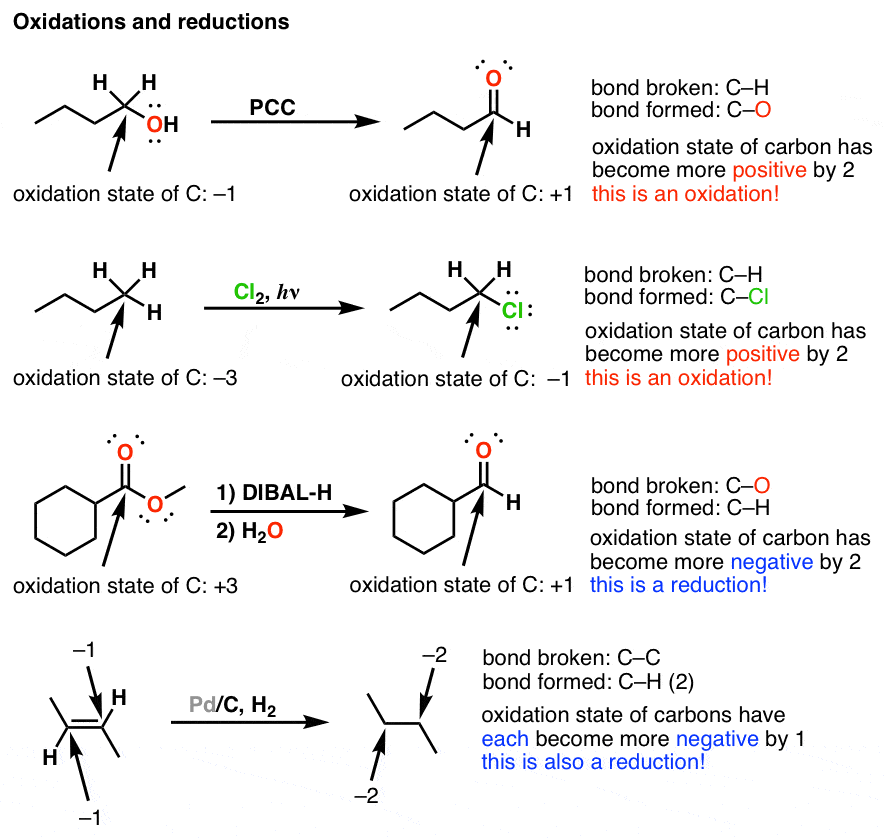
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry
These reactions need organic matter bacteria and no oxygen to be present in order to occur.
Oxidation and reduction atmospheres coloring pages. And in doing so gives up and electron. See more ideas about oxidation reduction redox reactions. One manages to create a reducing atmosphere in a kiln by reducing combustion air or by increasing fuel flow.
With fuel-burning kilns however care must be taken to ensure that the kiln does not go into reduction until the latter part of the. The copper that is the primary component of the statue slowly underwent oxidation from the air. The parallel exists in aqueous environments.
However oxidation can also be an effect of photochemistry initiated by stellar UV radiation. These include single replacement combustion and combination. The two processes always go together.
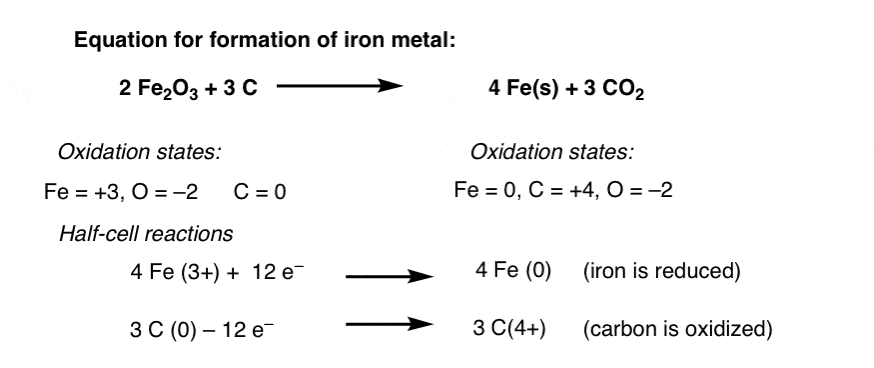
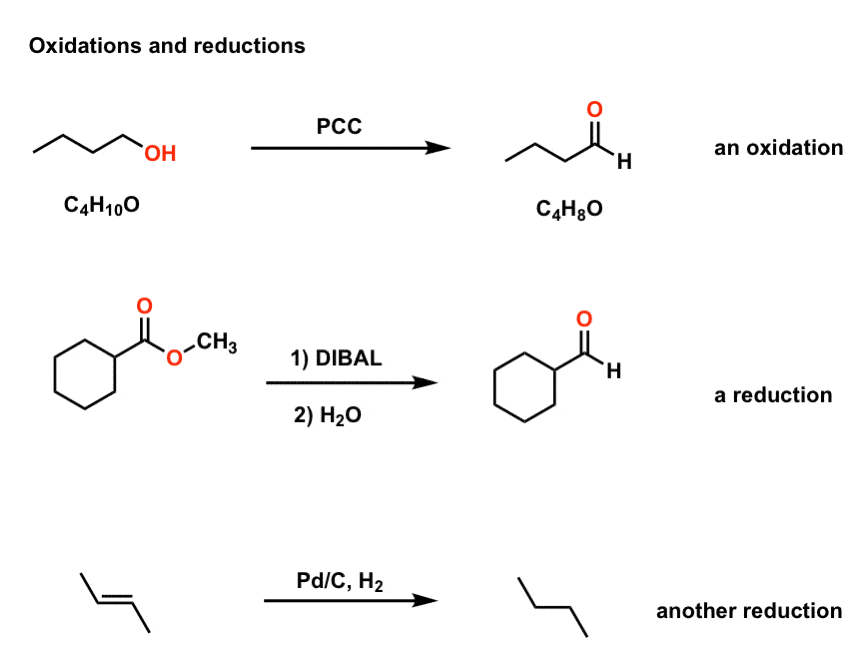
Lets learn how to assign oxidation numbers. Oxidation reduction reactions are very valuable in organic synthesis. Oxidation is the reaction in which the metal combines with some other element oxygen sulfur chlorine etc.
Oxidation Number Oxidation numbers are a useful tool for determining whether a substance has been oxidized or reduced. Notice the grey color of reduction vs. However some sandy soils are naturally low in iron and have low chroma colors not related.
In oxidation excess oxygen attaches itself to the surface of the glaze and clay. Chem 112 OXIDATION-REDUCTION EXPERIMENT. The excess oxygen burns through the metal.
Gleason ball clay fired test bars from cone 7-11 oxidation and cone 10 reduction. The main point being to arrive at the good moment at the good temperature and with a reasonable rate of unburn. It can also give pottery a speckled appearance as flecks of iron in the clay peep through the glaze.
Carbon monoxide is the reducing gas which is easiest to produce in the range of temperature of traditional ceramics. The change in appearance was a direct result of corrosion. Recognizing whether a compound is oxidized or reduced is an important first step in being able to successfully choose the correct reagents in a chemical transformation.
Feb 26 2016 - Explore Stephanie Bes board oxidation reduction on Pinterest. The stability of lithium aluminate LiAlO 2 powders have been studied under reducing 4 H 2 N 2 and oxidizing Air atmospheres at 700 CWhile XRD results show the raw α-LiAlO 2 samples contain a minor fraction of LiAl 2 OH 7 2H 2 O and LiAlO 2 025H 2 O phases high-temperature XRD study shows that LiAl 2 OH 7 2H 2 O may have decomposed at 700 C to γ-LiAlO 2 and LiAlO 2 0. COLTON203 IT IS A well known fact that the surface color of pottery made by the ancient inhabitants of the Southwest occurs 1 in shades of white and gray and also 2 in shades of red yellow and brown.
Electric kilns are naturally in an oxidation or neutral atmosphere. A less extreme example might be a pottery kiln where an oxidizing atmosphere keeps metal oxide pigments from giving up their. Oxidizing atmosphere refers to a gaseous atmosphere in which an oxidation reaction occurs usually the oxidation of solids.
Each oxidation is accompanied by a reduction and each reduction is accompanied by an oxidation. The yellowish of oxidation. The amount of low chroma color in soil is usually related to how long it has been reduced and not how long it has been saturated.
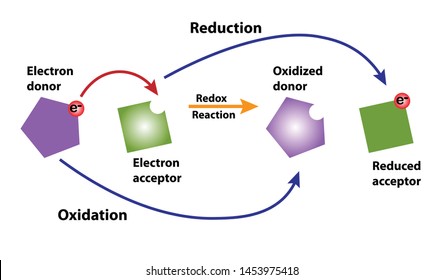
Oxidizing and Reducing Agents. An element that undergoes a change in oxidation number in the course of a reaction has been oxidized or reduced. The most dramatic of these is copper which changes from green to red.
THE REDUCING ATMOSPHERE AND OXIDIZING ATMOS-PHERE IN PREHISTORIC SOUTHWESTERN CERAMICS HAROLD S. The oxidation-reduction reactions of copper metal in the environment occur in several steps. Another web page but not for KS4-GCSE.
This is why oxidation and reduction are usually referred to as redox reactions - the term helps to emphasize this fact. It was brown the color of its copper skin So how did the Statue of Liberty change colors. The oxidation bars lower are typical of most ball clays.
The best example of this is an oxyfuel cutting torch. An oxidizing atmosphere is a planetary atmosphere which oxidizes immersed surface compounds. Many reactions that you have already studied are redox reactions.
Oxidation firing creates bright clean colors. So Im not trying to say that my oxidation-fired pots look just like they would in reduction but rather that they have all of the subtleness and variation that they once had when fired in reduction and that many of them show no appreciable difference. Reduction is the same thing in the opposite direction.
Oxidationreduction reactions are mostly responsible for gray colors in soils. An oxidation-reduction redox reaction involves the movement of electrons from one reactant to another. Recognizing Oxidation and Reduction of.
Oxidation is the loss of electrons. Oxidation and Reduction in Terms of Firing Schedules. Some coloring oxides change color when fired in reduction.
It sometimes refers to an O 2-rich atmosphere for example the atmosphere of modern EarthMost of the Earths atmospheric oxygen is generated by biological photosynthesis. A metal being corroded or the site on the surface being attacked is the anode.
Redox High Res Stock Images Shutterstock
Redox High Res Stock Images Shutterstock
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry
Redox High Res Stock Images Shutterstock
Pin On Inorganic Organic Chem
Pin On Chemistry
Pin On Analytical Chem
Redox High Res Stock Images Shutterstock